Gut check: Evaluating the effects of a ketogenic diet on athlete intestinal health
By Alex Ritson MSc, Head of Education

Research: The Impact of a Short-Term Ketogenic Low-Carbohydrate High-Fat Diet on Biomarkers of Intestinal Epithelial Integrity and Gastrointestinal Symptoms by McKay et al. (2023)
Introduction
Injuries and other visible ailments (think flu-like symptoms) often come to mind when considering common obstacles that impede an athlete’s performance. Yet, the private, sometimes mortifying, challenges of exercise-induced gastrointestinal (ExGI) symptoms (think bloating, gastric acidosis, flatulence, diarrhoea, and nausea) can be just as pervasive and debilitating for the athlete. While ExGI symptoms can occur in most sports (e.g., strength and power, team sports and short-duration endurance), it’s athletes competing in long-duration (>2 hrs) endurance sports that are most susceptible (Costa et al., 2022).
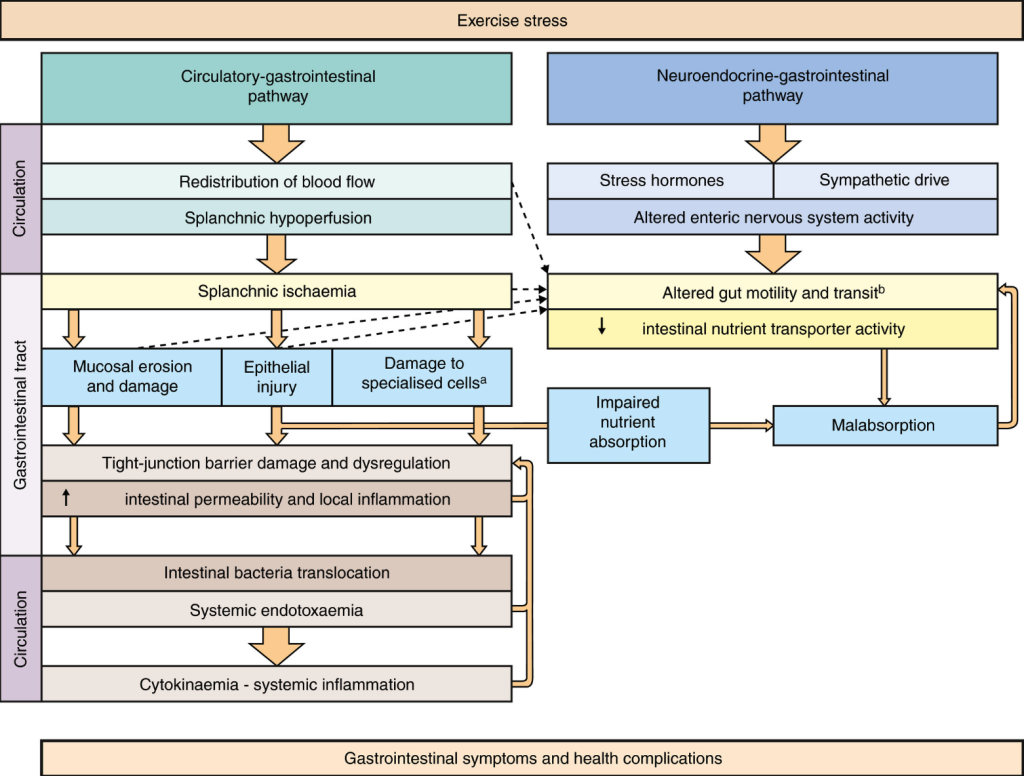
According to Costa et al. (2017), these symptoms stem from two interconnected pathways: the circulatory-gastrointestinal and neuroendocrine-gastrointestinal pathways, defined as exercise-induced gastrointestinal syndrome (Costa et al., 2017). As illustrated in Figure 1, during high-intensity, long-duration, endurance exercise, oxygen-rich blood is redistributed from the splanchnic region (e.g., stomach, small intestine, large intestine, liver, spleen, and pancreas) to the working muscles and heart. In addition, cortisol levels rise, and the stimulatory neurons of the GI tract dial down their activity, resulting in delayed gastric emptying, less secretory activity, and fluid exchange. These series of events are terrible news for the intestinal epithelium, which refers to the layer of cells that line the surface of the intestines. The intestinal epithelium serves multiple functions, such as acting as a gateway for nutrient absorption or barrier to harmful substances (e.g., pathogens and toxins) entering circulation. Moreover, it secretes mucus, enzymes and hormones that facilitate digestion; plays a vital role in immune function; and serves as a habitat for gut microbiota. The pathways of exercise-induced gastrointestinal syndrome result in intestinal epithelial cell injury and increased permeability. This means bacteria and their toxic by-products can enter circulation —this is known as ‘leaky gut’. Once bacteria and their endotoxins enter the blood, this triggers an immune response, resulting in local and systemic inflammation. This inflammatory response can contribute to ExGI symptoms and potentially impair performance, as well as cause other health concerns (Costa et al., 2022).
Several studies have explored the impact of carbohydrate (CHO) intake (amount and total) on intestinal epithelial function during exercise (King et al., 2022; Snipe et al., 2017). However, the effects of a low-CHO, high-fat diet (e.g., a ketogenic diet) or low energy availability (low EA) state — both conditions, either by design or accident, are commonly adopted by endurance athletes — have yet to be explored. This study aimed to assess and compare the impact of three short-term (6-day) dietary conditions: High-CHO, low-CHO, high fat, or low EA diets on biomarkers of intestinal epithelial health following high-intensity, long-duration exercise.
Figure 1. Exercise-induced gastrointestinal syndrome. Retrieved from Costa et al. (2017)
Helpful hint: An analogy for conceptualising how the intestinal epithelium functions
The intestinal epithelium and its roles can be hard to conceptualise. For those who like analogies, think of it like an international airport’s check-in area. A healthy intestinal epithelium acts like a diligent check-in officer, carefully inspecting and regulating what ‘passengers’ (e.g., nutrients) get checked in and what ‘unauthorised travellers’ (e.g., pathogens and toxins) get denied entry. The tight junctions between intestinal epithelial cells resemble the security screening areas. They ensure the safety of the internal environment by allowing only passengers with the right authorised baggage through. The mucus produced from the intestinal epithelium is like the security conveyor belt, ensuring the smooth and efficient transition of materials (digested contents). Like an airport’s check-in area, the intestinal epithelium function varies depending on dietary inputs (e.g., number of passengers wanting to fly) and other factors, such as blood flow (e.g., staff numbers) and sympathetic drive (the communication system). During high-intensity, long-duration exercise (particularly in hot environments), it’s like the airport check-in area being short-staffed and technical glitches occurring with the communication system. This results in check-in officers receiving fewer updates, causing long queues (e.g., delayed gastric emptying). Due to the airport being short-staffed (e.g., lack of blood flow), security checks become less stringent (e.g., tight junctions loosen), and the conveyor belt slows down (e.g., less mucus secreted). The net result is security threats (e.g., pathogens and toxins) entering the departure area (e.g., absorbed into the bloodstream), causing panic among security staff (e.g., inflammation), flight delays (e.g., impaired digestion/absorption), and disgruntled passengers (e.g., GI symptoms).
The study:
This was a non-randomised, parallel-group study design. Twenty-eight male race walkers were recruited with a training status defined as ‘World-Class’ (n=4), ‘Elite’ (n=20), and ‘Highly Trained’ (n=4), according to McKay et al. (2022) (see this review). For the first six days of the trial (baseline period), all athletes adhered to a high CHO diet with optimal EA before completing a 25-km race walk. Following the baseline period, they were split into three groups according to their dietary preference: a high CHO diet with optimal EA (65% CHO, 15% protein, 20% fat; 40 kCal.kg FFM⁻¹.day⁻¹), a low CHO, high-fat diet with optimal EA (<50 g of CHO, 15% protein, 80% fat; 40 kCal.kg FFM⁻¹.day⁻¹), and a low EA condition (60% CHO, 25% protein, 15% fat; 15 kCal.kg FFM⁻¹.day⁻¹). Athletes adhered to these diets for 6-days before a second 25-km race walk.
For the first race day, all athlete’s pre-race breakfast consisted of 2g.kg.BM of CHO. For the second 25-km race, athletes in the low-CHO, high-fat group consumed a low-CHO, high-fat energy-matched meal (to the high CHO condition), the low-EA group consumed 1g.kg.BM of CHO, and the high-CHO group replicated their baseline intake.
During the first race, all athletes received CHO sports gels (60g/hr). For the second 25-km race walk, the high CHO group maintained the same dietary intake as the first race, while the low-CHO high-fat group received high-fat snacks, and the low-EA group consumed sports gels at half the quantity (~30g) of the high-CHO condition.
Before, during, and after both 25 km-race walks, biomarkers of epithelial injury were sampled: a protein found in the lining of the intestine (intestinal fatty acid-binding protein; I-FABP) and indirect markers of bacterial infection (lipopolysaccharide-binding protein; LBP, and soluble CD14). In addition, gastrointestinal symptoms were assessed via a 10-point visual analogue scale.
Findings:
A marker of intestinal epithelial injury (I-FABP) was two-fold higher in the low-CHO, high-fat condition compared to baseline. In addition, an indication of bacterial infection (soluble CD14 and LBP) was 53% and 36% higher in the low CHO, high-fat group compared to baseline. Comparatively, there were no significant differences in the high CHO or low EA groups compared to baseline. However, the changes in blood markers did not correspond with alterations in GI symptoms, with incidences generally remaining the same between conditions. With that said, there was a nonsignificant increase in upper GI symptoms (think belching, bloating, urge to regurgitate) in the low-CHO, high-fat condition compared to baseline and other groups.
While these secondary findings on performance have been documented elsewhere (Burke et al., 2021), it’s worth mentioning how the athletes adopting the low-CHO, high-fat diet demonstrated a significant drop in their performance times during the 25 km race walk compared to baseline. Specifically, these athletes took a further ~7.5 mins to complete the 25-km race walk compared to when they adopted the high CHO diet. The slower race times were due to athletes racing at a self-chosen lower exercise intensity (-6% lower percentage of their V̇O2 max); yet they showed signs of increased physical exertion (athletes reported a ~10 beats per minute increase in heart rate compared to baseline).
Relevance:
To recap, this research aimed to assess the effects of different diets (high-CHO, low-CHO, high-fat and low EA) on biomarkers of intestinal epithelial injury following exercise. Adopting a low-CHO, high-fat diet for 6 days resulted in a two-fold increase in markers of intestinal epithelial damage and signs of bacterial infection. Conversely, adherence to a high-CHO or low-EA diet resulted in no significant changes to intestinal epithelial function. These results suggest that adopting a low-CHO, high-fat diet in the short term may compromise the function of the intestinal epithelium, increasing the risk of leaky gut and bacterial infection. Returning to the intestinal epithelial analogy, adopting a low-CHO, high-fat diet might be like the airport’s check-in area being filled with disruptive passengers, agitating the check-in officer, and distracting other airport staff, impairing its normal regulatory function. The result is unauthorised individuals slipping past security and causing severe problems in the departure area. While the increase in biomarkers of intestinal epithelial injury didn’t translate to significant changes in GI symptoms, these results should still raise alarm bells for athletes’ susceptible to GI symptoms or athletes competing in long-duration, high-intensity endurance events (> 2), particularly in hot environments. From a performance perspective, adopting a low-CHO, high-fat diet significantly reduced race times due to athletes working at a reduced level of their V̇O2 max yet exerting greater physical effort. In other words, the athletes needed to produce more energy to sustain the same pace/power for a given V̇O2 max. This is like switching a high-performance car’s fuel from high octane fuel (high CHO), which burns quickly and efficiently for optimal speed/power output, to standard diesel (low-CHO, high-fat), which has a higher energy content per litre but burns slower and less efficiently. Take home, carbs are king when athlete’s need to train and compete at their optimum.
Complement this research with the We Do Science podcast episode: Episode 143: The athlete’s gut with Dr Patrick Wilson
References:
Burke, L. M., Whitfield, J., Heikura, I. A., Ross, M. L. R., Tee, N., Forbes, S. F., Hall, R., McKay, A. K. A., Wallett, A. M., & Sharma, A. P. (2021). Adaptation to a low carbohydrate high fat diet is rapid but impairs endurance exercise metabolism and performance despite enhanced glycogen availability. The Journal of Physiology, 599(3), 771–790. https://doi.org/10.1113/JP280221
Costa, R. J. S., Snipe, R. M. J., Kitic, C. M., & Gibson, P. R. (2017). Systematic review: Exercise-induced gastrointestinal syndrome-implications for health and intestinal disease. Alimentary Pharmacology & Therapeutics, 46(3), 246–265. https://doi.org/10.1111/apt.14157
Costa, R. J. S., Young, P., Gill, S. K., Snipe, R. M. J., Gaskell, S., Russo, I., & Burke, L. M. (2022). Assessment of Exercise-Associated Gastrointestinal Perturbations in Research and Practical Settings: Methodological Concerns and Recommendations for Best Practice. International Journal of Sport Nutrition and Exercise Metabolism, 32(5), 387–418. https://doi.org/10.1123/ijsnem.2022-0048
King, A. J., Etxebarria, N., Ross, M. L., Garvican-Lewis, L., Heikura, I. A., McKay, A. K. A., Tee, N., Forbes, S. F., Beard, N. A., Saunders, P. U., Sharma, A. P., Gaskell, S. K., Costa, R. J. S., & Burke, L. M. (2022). Short-Term Very High Carbohydrate Diet and Gut-Training Have Minor Effects on Gastrointestinal Status and Performance in Highly Trained Endurance Athletes. Nutrients, 14(9), Article 9. https://doi.org/10.3390/nu14091929****
Snipe, R. M. J., Khoo, A., Kitic, C. M., Gibson, P. R., & Costa, R. J. S. (2017). Carbohydrate and protein intake during exertional heat stress ameliorates intestinal epithelial injury and small intestine permeability. Applied Physiology, Nutrition, and Metabolism, 42(12), 1283–1292. https://doi.org/10.1139/apnm-2017-0361